Authors: Abigail Burdon, Thomas Jaki, Xijin Chen, Pavel Mozgunov, Haiyan Zheng, Richard Baird
Published on: May 10, 2024
Impact Score: 7.6
Arxiv code: Arxiv:2405.06353
Summary
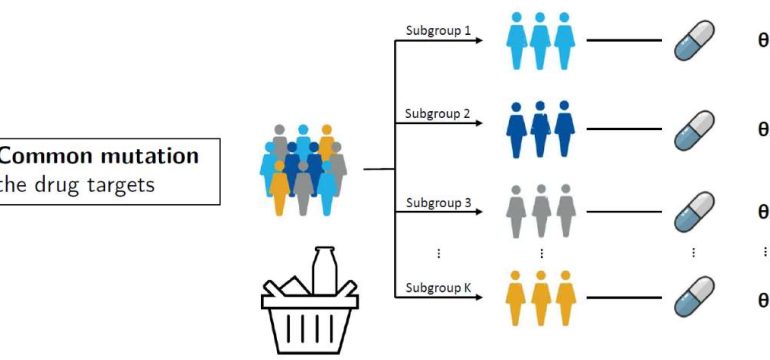
- What is new: Introduction of seamless clinical trials and master protocols to streamline drug development in cancer research.
- Why this is important: Inefficiency, high costs, and lengthy processes in drug development, especially in Phase II / III cancer clinical trials.
- What the research proposes: Utilizing seamless clinical trials and master protocols to accelerate the development of promising therapies and investigate multiple treatments within a single trial.
- Results: These methods have shown success in accelerating therapy development and addressing regulatory considerations, ultimately aiming to deliver treatments to patients faster.
Technical Details
Technological frameworks used: Seamless clinical trials, Master protocols
Models used: Not specified
Data used: Real trial examples
Potential Impact
Pharmaceutical industry, specifically companies involved in oncology drug development
Want to implement this idea in a business?
We have generated a startup concept here: TrialStream.

Leave a Reply